half life formula chemistry
In which N 0 is the number of atoms you start with and N t the number of atoms left after a certain time t for a nuclide with a half life of T. Determining a Half Life.

Radioactive Decay And Half Life Chemistry Lessons Chemistry Classroom Chemistry Lesson Plans
A specific isotope might have a total count of 30000 cpm.

. So the half-life of that isotope is one hour. N t N0. By putting these values in equation i we get.
If an archaeologist found a fossil sample that contained 25 carbon-14 in comparison to a living sample the time of the fossil samples death could be determined by rearranging equation 1 since N t N 0 and t 12 are known. T time interval t 12 for the half-life. Your half-life of a first.
T ½ 1 k A o Top. Then half-life t 12 2λ. The half-life of fluorine-20 is 110 s.
The formula for half-life in chemistry depends on the order of the reaction. T ½ A o 2k For a first order reaction A products rate kA. 789 h o u r s.
The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value. Calculate the half-life of Gold-198 given that 3257 mg of this radioactive isotope decayed to 102 mg in 135 days. An ingenious application of half-life studies established a new science of determining ages of materials by half-life calculations.
Where N0 refers to the initial quantity of the substance that will decay. 2λ 0693 λ. Other isotopes have shorter half-lives.
One can describe exponential decay by any of the three formulas. Then write the half-life equation as. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2.
You can replace the N with the activity Becquerel or a dose rate of a substance as long as you use the same units for N t and N 0. Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial value. We know that at the half-life time eqt_12 eq the concentration of the reactant will be half as much as the initial concentration.
We use the equation A t 1 2 t t 1 2 A o where A t is the activity in time t A o is the original activity 500 1 2 10 t 1 2 6000 t 1 2 10 log 2 log 12 2. Given that for a First Order reaction the half-life is twice the value of the rate constant find the value of the rate constant of the reaction. If k is a constant obviously 693 is a constant.
λ 2 03465. And for the second-order reaction the formula for the half-life of the reaction is given by 1k R 0. If a sample initially contains 500 g of fluorine-20 how much remains after 600 s.
T is the half-life. T 12 0693 λ. This means our y-axis values will be as follows.
For a zero order reaction A products rate k. For a zero order reaction the formula is t½ Ao 2k. Where t12 is the half-life of a certain reaction unit - seconds R0 is the initial reactant concentration unit - molL-1.
Half-life can also be expressed interms of the number of half-lives n and total time t as in the equation below. Solution t 1 2 13. The general equation with half life.
We can determine the amount of a radioactive isotope remaining after a given number half-lives by using the following expression. Activity or decay constant. In this case we know that in 20.
K decay constant. K is the rate constant. 2λ 2 0693.
This means that the fossil is 11460 years old. Another equation you might. Min H1ê2Ln Nn ÅÅÅÅÅÅÅÅÅÅ N0 010 N0 ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ N0 010.
We can also use the relation A t 1 2 n A o where n is the number of half-lives A t A o 2 n. For the first-order reaction the half-life is defined as t12 0693k. Some isotopes have long half-lives the half-life of U-234 is 245000 years.
Graphical relations and half lives. This expression works best when the number of half-lives is a whole number. It is also possible to determine the remaining quantity of a substance using a few other parameters.
The measurement of this quantity may take place in grams moles number of atoms etc. Now lets think about this. Ln N N o 2 k t 12.
N t N 0 05 t T. The decay constant is the amount of radioactive substance that disintegrates in a unit of time. Let the rate constant be λ.
For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao. It is a constant and related to the rate constant for the reaction. Here we identify the initial amount as 500 g t 600 s and t 12 110 s.
2 270 days. N t mass of radioactive material at time interval t N 0 mass of the original amount of radioactive material. For geological dating the decay of U -238 can be used.
T 12 0693k. 25 125 625 3125 15625. So our half-life is equal to let me rewrite this here so our half-life t 12 is equal to 693 divided by k where k is our rate constant.
Get access to. The end product of the decay of U. T 1 2 Half life of the substance.
Where t 12 is the half-life time. What is its half-life. Therefore we can set eqA eq equal to eqA_02.
λ 0. 5 log 2 log 325. Ln 2 k t 12.
And so your half-life is constant. In one hour the count could be 15000 cpm half the original count. So here is your half-life for a first order reaction.
Although similar to Example 3 the amount of time is not an exact multiple of a half-life. Calculate the half-life of the radioactive source. Solving for n we get-n logH2LlogH010LlogH10μ10-1L-1 n têthalf 1êlogH2L1ê03020 minêthalf.
N t N0. The half-life of U -238 is 45 109 years. So we have the negative of that so we get a positive value here for our half life.
T 12 is the half-life. The half-life of a first-order reaction does not depend upon the concentration of the reactant. For each half-life that occurs the amount of In-115m decreases by half of the previous point.
As always lets begin with the fundamental expression Nn H1ê2Ln N0. T 12 is the half-life τ is the mean lifetime λ is the decay constant. N t N 0 e -tτ N t N 0 e -λt τ is the mean lifetime - the average amount of time a nucleus remains intact.
Equations for Half Lives. Where n is the number of half-lives. Substituting into the equation.
The half-life of fluorine-20 is 110 s. N t N0. At half-life time t t 12 N N o 2.

Chapter 6 Oxidation Reduction Redox Reactions Teaching Chemistry Chemistry Study Guide

Calculate The Number Of Stereoisomers Chemistry Basics Chemistry Paper Chemistry Study Guide

Half Reaction Easy Science Redox Reactions 10th Grade Science Cool Science Experiments

Condensing Logs Logarithmic Functions Functions Math Organic Chemistry Study

Chemical Kinetics And Half Life Online College Chemistry Courses Chemical Kinetics Study Chemistry Half Life

Half Life Practice Worksheet Answers A Worksheet Is Often A Small Note Distributed By A Tutor To Students Tha Half Life Persuasive Writing Prompts Worksheets

Zero Order Kinetics Reactions Online College Chemistry Courses College Chemistry Online Chemistry Courses Physical Chemistry

Kinetics Ppt Chemical Kinetics Chemical Equation Enzyme Kinetics

Zero Order Kinetics Chemistry Textbook Chemistry Classroom Chemistry Experiments

Half Life Calculator Real Life Math Half Life Learning Techniques

Radioactive Decay Formula Radioactive Half Life 0 693 Radioactive Decay Constant Physics Topics Science Themes Calculus
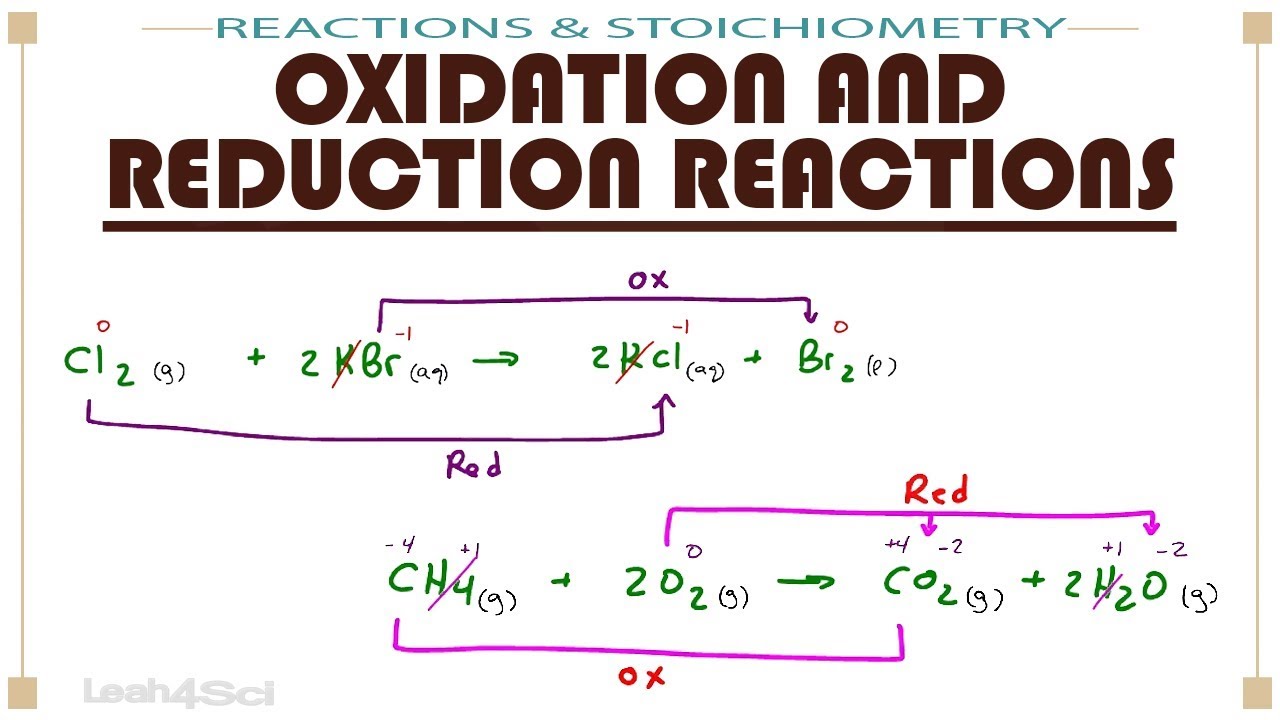
Learn How To Recognize Redox Reactions Oxidation Species Reduction Species And Half Reactions Mcat Chemistry P Redox Reactions Mcat Study Ap Chemistry

Atomic Size Order Chemistry Math Atom

As Level Physics Formula Sheet Physics Formulas Physics Lessons Physics Facts

Ch 5 5 Multiple Angle And Product To Sum Formulas Ppt Download Free Math Help Trigonometry Word Problem Worksheets

Calculation Of Half Life Of Radioactive Substances Half Life Physics Formulas Life

Quantum Number Orbital Shell Chemistry Classroom Science Chemistry Study Chemistry

Basic Trigonometry Formulas Math Formula Chart Trigonometry Math Formulas
